A Race Against Time: Dean’s Lecture Series Explains AstraZeneca’s Efforts to Deliver 3 Billion Doses of COVID-19 Vaccine
Stevens alumna Pam Cheng '92 M.Eng. '95 reveals how, through her company’s efforts at top-speed around the clock, we can expect a safe and effective vaccine in early 2021
With the strike of COVID-19, 2020 has been an unprecedented year. Scientists and engineers around the globe have mobilized like never before to study the novel coronavirus and work toward a singular common goal: provide the world with a potentially life-saving vaccine.
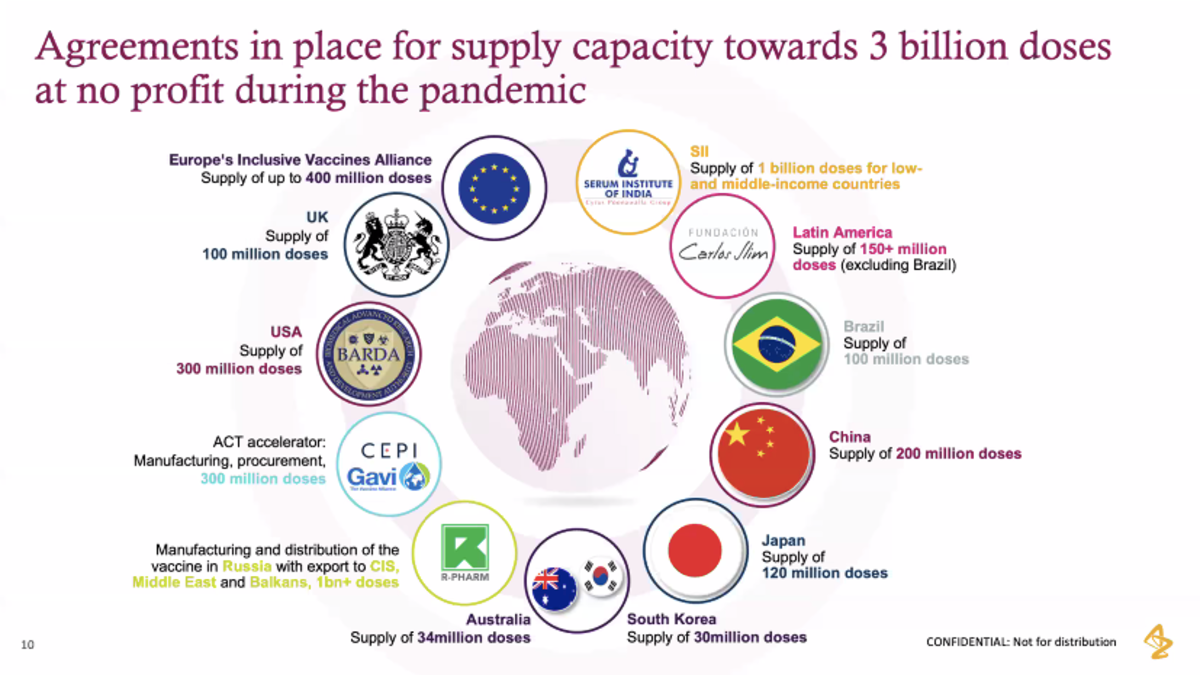
Stevens alumna Pam Cheng '92 M.Eng. '95, executive vice president of AstraZeneca and this year’s Dean’s Lecture Series guest speaker, delivered a hopeful message in her presentation titled “Fighting COVID-19 with Resilience — A Race Against Time”: The biopharmaceutical company is currently conducting phase III clinical trials of a vaccine against COVID-19, with an aim to have the vaccine available in early 2021, and plans to deliver three billion doses around the world at no profit. The vaccine, Cheng promised, will not be exclusively for the privileged or for wealthy countries, as the company is cooperating with governments around the world to deliver the vaccine at-cost — which is anticipated to be less than $10 per dose.
With AstraZeneca’s goal to cover half the world’s population’s doses, and multiple other companies joining their efforts to develop vaccines as well, today there is real hope that the course of the pandemic will be altered. While the post-pandemic world will undoubtedly be forever changed, the promise of a better future is within reach.
Disrupting life as we know it
“We are absolutely living in an unprecedented time, and we are living our lives very differently than [we were] seven months ago,” said Cheng during her presentation on October 21. “We are now speaking of new norms and wondering what life after the pandemic will be like.” With nearly 40 million people worldwide infected by the deadly virus, and over 1.1 million dead since its outbreak in December 2019, no part of the world has been left untouched.
“I think we have all been underestimating the complexity of the problem,” said Cheng. “The pandemic has gone beyond just being a health crisis. It has sparked fears of an impending economic crisis and recession. Social distancing, self isolation, and travel restrictions have led to a reduced workforce across all economic sectors, and has caused many jobs to be lost. Schools are now online; care centers have closed down. They are becoming a significant hindrance for productivity and raising learning gaps around the world. Central banks [have] globally committed to a whatever-it-takes approach in an attempt to save the global economy. The road to economic recovery is predicted to be a very long one, with a period of economic inactivity for years to come.”
She explained that supply shortages have not only resulted from panic-buying and stockpiling of basic products like foods and necessities; there have also been stockpiling of some medications which, if not properly managed, could lead to critical shortages across the world.
Cheng believes that innovation is key in the fight against COVID-19’s supply chain disruption, citing examples of ingenuity at work across the world, including the 3D printing of medical devices and the use of nontraditional parts to build ventilators.
During this crisis, AstraZeneca has maintained two top priorities: ensuring that medicine is supplied to all patients, and keeping their employees safe. They have been meeting these challenges despite shortages from key suppliers, many of whom are experiencing reduced workforces and surges in demand.
No time to spare: Closing the gap between discovery and market
AstraZeneca is tackling the virus from three primary fronts: the development of a potential vaccine through a collaboration with the University of Oxford, the development of a potential monoclonal antibody, and investigating potential treatments using both new and existing medications. “We are looking at the entire value chain, end-to-end from virus entry to potential treatment for someone who is infected,” Cheng said.
While the development and manufacturing of vaccines usually takes years to do — with an industry average of eight-to-ten years for discovery, development, manufacturing, and delivery to be completed—AstraZeneca is racing to have their COVID-19 vaccine ready and available in 2021.
“There is quite a bit of noise around whether we’re taking shortcuts, and I can tell you unequivocally that we are absolutely not taking shortcuts,” Cheng emphasized. “We are taking all the safety protocols. There’s nothing you can rush with this.”
A quick, but safe, delivery through collaboration and smart manufacturing
While all the usual discovery and development protocols are being stringently followed, the part of the process that AstraZeneca is accelerating is the manufacturing aspect and shaving years off of the time-to-market.
“Typically what happens is we will not invest in manufacturing until we have data from the clinical trial that it’s an effective and safe vaccine—because that investment is north of a billion dollars,” said Cheng. “We have partnered with governments around the world and many foundations to invest at-risk in manufacturing before we really know if it’s a safe vaccine. This is how we’re able to accelerate without taking any shortcuts. Our goal here is, when the FDA (Food and Drug Administration) approves the vaccine, we will have commercial doses available, and we will have not lost a single day.”
Recognizing at an early point that a vaccine would be necessary to combat the virus, AstraZeneca undertook the seemingly impossible toward this goal: converting a massive building known as a sterile powder filling asset into a biosafety containment facility dedicated to the formulation, filling, and packaging of a live virus vaccine at record speed. What would normally be an 18-month project, they delivered in just five months by repurposing existing machinery and bringing the engineering and design in-house. Cheng called this feat a “smart factory.”
“If you had asked me [before the pandemic] if I could build this kind of facility in less than five months—no way,” She said. “It’s amazing when you can combine innovation, technical skill sets, and a common goal… Wonderful things happen.”
Safety first: Vaccine trials demystified
The clinical development of a vaccine is a three-phase process. In the first phase, the vaccine is given to small groups of people; second, the vaccine is given to people who have characteristics similar to those for whom the vaccine is intended; and finally, the vaccine is given to thousands to test for efficacy and safety.
AstraZeneca currently has phase III trials ongoing in multiple countries, with tens of thousands of volunteer subjects participating.
Cheng explained that in their clinical trials, they inject healthy human subjects with the vaccine and monitor for robust immune responses against the virus. So far, they are seeing rapid antibody and T-cell responses; and neutralizing antibodies in 91% of participants one month after the first dose, and in 100% after the second dose.
Cheng stated that they look at four aspects of a vaccine as they evaluate it: a strong immune response, T-cell response, neutralizing antibody activity, and safety. “We are seeing very robust protection,” she said. She added that she hopes for multiple vaccines from multiple companies.
Ethically prioritizing vaccine distribution
Fair and equitable supply of the vaccine is a major global concern over the coronavirus crisis and it’s also a key priority of AstraZeneca.
“We will provide broad and equitable access of this vaccine around the world. This will not be a vaccine for the privileged; this will not be a vaccine just for the wealthy countries,” Cheng promised. “We have committed to supply this vaccine at no profit during the pandemic, so we are not in this to make a commercial benefit; we are in this because we believe it’s the right thing to do. We believe this is the time where we step forward to make a difference in this pandemic. We remain true to that today, and we will continue to work with governments and other organizations around the world to ensure fair and equitable supply.”
A Stevens education emphasizes solving the world’s toughest problems
Cheng holds bachelor’s (’92) and master’s (’95) degrees in chemical engineering from Stevens, and an MBA in marketing from Pace University. In 2019 Cheng was honored at the 6th Stevens Awards Gala, where she received the International Achievement Award.
Of her Stevens education, she said, “I wouldn’t be who I am if I didn’t go to Stevens… What Stevens taught me was this ability to solve problems, having that resilience. You see a problem, you don’t shy away from it—you solve it. Being creative, being innovative, and problem-solving skills [have stayed] with me through every job I have had in my career. That problem-solving, innovative mindset helps you no matter what kind of job you take in the future.”
Cheng joined AstraZeneca in 2015 as Executive Vice President of Global Operations and Information Technology, guiding the company’s manufacturing, supply chain, procurement, and IT across 18 countries and leading a team of over 19,000. Before AstraZeneca, she spent 18 years in global manufacturing, supply chain, and commercial roles at Merck/MSD.
“It is our great pleasure to have Ms. Cheng as our distinguished speaker this year,” said Dean Jean Zu of the Charles V. Schaefer, Jr. School of Engineering and Science. “None of us could have foreseen the great difficulties our Stevens community along with the whole world would face in 2020. I am heartened to see Ms. Cheng’s innovative efforts against COVID-19, at a time when her leadership is more important than ever. I am proud to call Ms. Cheng both a Stevens alumna, and a friend.”
Today, Cheng has two daughters enrolled at Stevens.
Looking beyond COVID-19
Life after COVID-19 is a much-speculated topic as the global impact continues to unfold. As for Cheng, she envisions a world forever changed by the pandemic that has brought so much collective uncertainty and loss.
“There’s a lot we don’t know, but one thing we do know is that life will be very different after the pandemic,” she said. “I continue to believe innovation and resilience will be key moving forward. Digital and virtual will be here to stay. We will all travel less. Flexibility will increase in how we work, where we work, and when we work. Network resilience and remote connectivity will be more important than ever; along with it—cybersecurity. However our world will change, our industry and our company will remain focused on advancing drug discovery and the delivery of innovative medicines and solutions to those in need.”
Learn more about the Department of Biomedical Engineering at Stevens:
Learn more about the Department of Chemical Engineering and Materials Science at Stevens: