Stevens researcher Stephanie Lee engineers green, portable, affordable power sources for a brighter future
Between the scarcity of fossil fuels and the rise in global temperatures, the U.S. is increasingly turning to environmentally friendly energy sources to power homes, businesses and infrastructure. Solar panels are extremely promising, but the ones currently available are clunky, expensive and inefficient.
That’s why Stephanie Lee is making better ones.
"Imagine a world in which installing solar panels is as easy as painting them on your roof," says Lee, assistant professor of chemical engineering at Stevens Institute of Technology. "That’s a world my students and I are working to create every day."
Solar panels like the ones used in industry or homes are made of silicon. They’re heavy, brittle, expensive to own and manufacture—and only convert between ten to twenty percent of absorbed sunlight into electricity. "We need better solutions," Lee says, "ones that can be produced fast enough to keep up with growing energy demand."
"Generating electricity anytime there is sunlight"
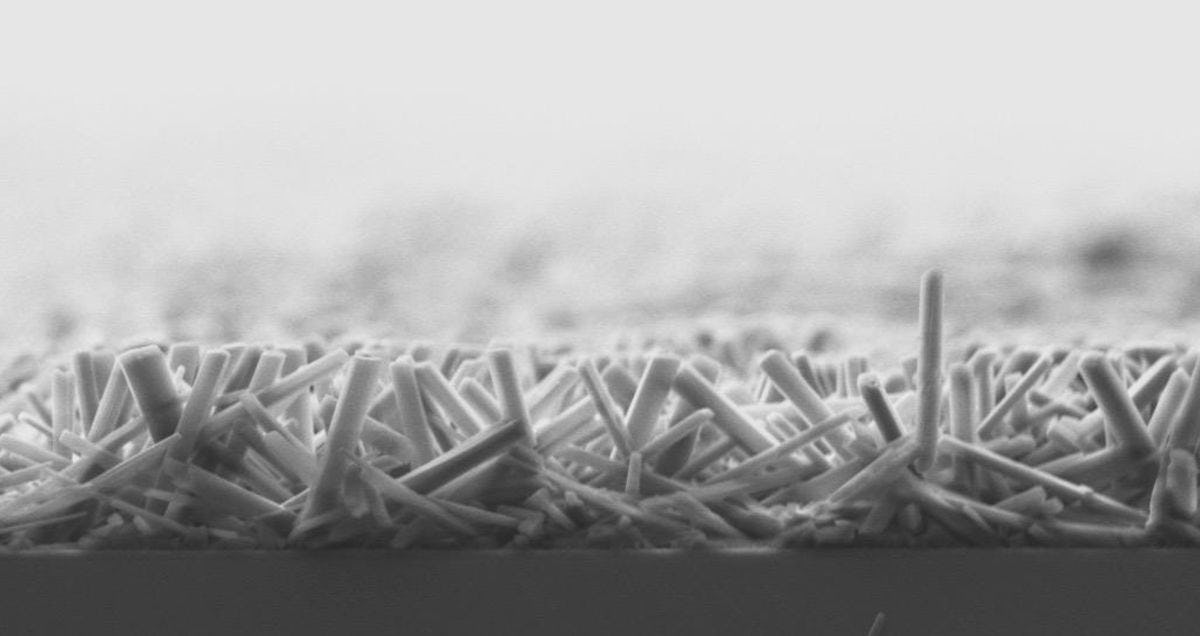
The solar panels Lee makes are made of perovskite, a material that absorbs light extremely well. Perovskite solar cells are ultra-thin, lightweight, portable, cheap to manufacture and so flexible "you can roll them up and put them in your backpack," Lee says. This means they can go anywhere and be used by anyone regardless of where they are—or what they can afford. "They could be used for electricity generation in remote locations, as wearable panels embedded in backpacks or clothing, or even as cell phones and screens," Lee says. "Because they are so portable, lightweight solar panels will allow us to generate electricity anytime there is sunlight. We don’t need to be constantly plugged in."
Perovskite is such a promising material that many researchers are creating solar cells with it. But it’s chemically unstable.
Thankfully, Lee and her team figured out a solution: "Our research is focused on improving the stability of perovskite solar cells by using anodic aluminum oxide nano-confinement," says Lee’s Ph.D. student Xiaoqing Kong. "Most commercial companies don’t use perovskite solar cells because when they’re exposed to oxygen and moisture they completely breakdown in 14 days. Our technique makes perovskite solar cells stable for up to two years."
Making green energy portable–and affordable–for everyone
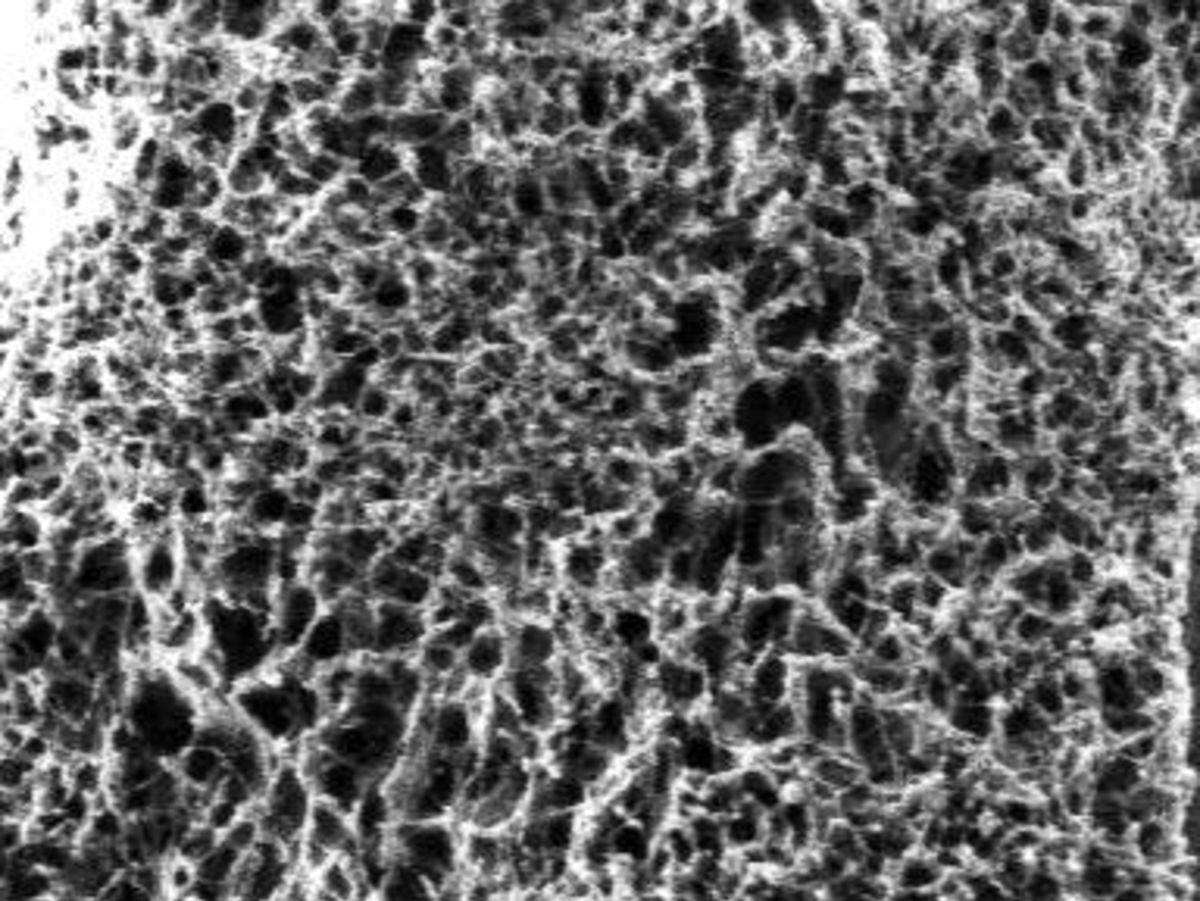
Lee and students in the LEE Lab make these cells by optimizing the microscopic material and spreading it like jelly across a thin plastic film to create panels. "The active components of solar panels are dissolved in solution and deposited over large areas through technologies such as inkjet printing and roll-to-roll coating," Lee explains. This method creates panels that can be manufactured quickly and effectively, driving down the cost of electricity generated from the sun and paving the way for commercial and residential use.
In order to make that process more friendly for mass production, Lee has teamed up with Dilhan Kalyon, professor of chemical engineering and material science, affiliated joint professor of biomedical engineering and the director of Stevens’ Highly Filled Materials Institute. They received a generous one-year National Science Foundation grant to study polymeric systems used to convert solar energy in flexible organic solar cells earlier this year.
"One of the biggest challenges in the field of emerging solar panels is scaling up from the laboratory bench to commercial manufacturing," Lee says. "Top-performing emerging solar panels are currently fabricated in research laboratories using energy-intensive methods that drive up the cost of these devices. We’re trying to bring that down."
Together with Kalyon's Ph.D. student Jing He, they discovered a creative way to form high-performance crystalline polymer gel networks through viscoelastic phase separation—a major step in overcoming that hurdle. "This process is robust and reliable, which is critical for manufacturing," Lee says. "Most importantly, these polymer networks can be deposited onto solar panel platforms via continuous coating methods," which makes them ideal for commercial production.
The process is so effective they filed a provisional patent on it.
"Stephanie is diligent and visionary," Kalyon says. "With low cost and high efficiencies these photovoltaics, with their remarkable ability to wrap around vehicles and structures, will help lower our carbon emission footprint while reducing energy costs."
"Maximizing efficiency and scaling up production on solar panels like these will mean we can theoretically meet the entire global energy demand with solar energy harvesting," Lee says. "Plus, panels like these can make green energy affordable for everyone while reducing carbon dioxide emissions contributing to climate change."
PSEG partnership helping recharge the battery industry
This relentless researcher has also expanded her scope to the field of solid-state batteries.
Thanks to the generous support of a partnership with Public Service Enterprise Group (PSEG), Lee, Kalyon and new Stevens assistant professor Jae Chul Kim are leveraging their collective and considerable expertise to design lighter, safer, more powerful batteries. These batteries use ion-conducting inks and that can be manufactured on a large-scale, at a low-cost.
This work is proving especially fruitful, as the team is "uncovering fundamental scientific principles governing the behavior of molecules at length scales undetectable by even the most powerful microscopes," Lee says. This means that they’re learning how to better conduct electricity to make more efficient batters—and how to make better materials.
"When solar energy is not accessible, we can use these batteries to power devices," Lee says.
Powering resilience and sustainability in today's research
Lee’s passion for sustainable technologies—and creating solutions to major societal needs—runs deep.
"When I got to MIT, it was amazing being surrounded by peers who were 'nerdy' like me and also had diverse interests and passions," she says. "I wanted to pursue a career that would benefit society in a tangible, impactful way, but after an unfortunate college experience involving rats in a drug delivery laboratory, I quickly discovered I was too squeamish to pursue medicine or biomedical engineering. I switched to a research group focused on sustainable energy technologies."
Today, Lee is a champion for girls like her. She works to empower girls and underserved students to explore STEM careers through diversity initiatives such as participating in the annual U.S. "Introduce a Girl to Engineering" event and partnering with New York City’s Center for All Abilities.
And she shows no signs of slowing down.
"I am grateful to have the opportunity to invest in young people’s lives," she says, "and develop technologies that can generate electricity to meet our increasing global energy demand without negatively impacting our environment."
Stevens Institute of Technology has a longstanding and deep commitment to resilience and sustainability. It is foundational to both the university and the Schaefer School of Engineering and Science.