Stevens Researcher Creating New Materials for Energy Sources of the Future
The energy sources of the future—next-generation materials for batteries, fuel cell membranes and supercapacitors — will need new materials to support them.
Stevens Institute of Technology associate professor Pinar Akcora is creating those materials.
Akcora, an NSF CAREER Award winner who runs the Soft Materials Lab in the Department of Chemical Engineering and Materials Science, works on multifunctional flexible nanomaterials for their uses in membranes for separation technologies, water purification, and ion-conducting polyelectrolytes.
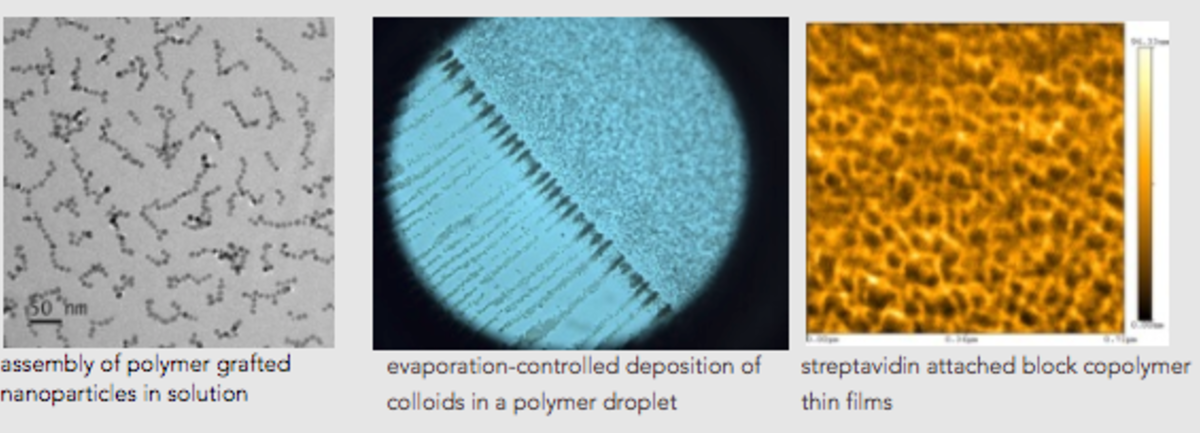
"My NSF CAREER project was on the self-assembly of multifunctional polymer-grafted nanoparticles," Akcora explains. After being awarded a grant from the National Science Foundation’s Division of Materials Research—Polymers program, she continues to design these particles for next-generation energy applications.
The NSF project, for which Akcora is the sole PI, is called "Ionic Transport in Ion Containing Copolymer-Grafted Nanoparticle Structures.” With this project, Akcora aims to design new, flexible and mechanically robust particle-based polymer electrolytes. The project aims to synthesize polymer functionalized spherical magnetic nanoparticles known as “polymer-grafted nanoparticles” by growing polymers with asymmetric composition, containing ion-containing polymer molecules in the end of chains. She plans to understand the relationship between nanoparticle structures in ionic liquids, -which are salts in liquid state-, and ionic conductivity. Her hope is to extend the functionality of this nanomaterial so they will be better suited to working with fuel cell electrolyte membranes.
"This new nanomaterial is leak- and vapor-free, and a non-flammable solid electrolyte with ion-conducting network structures," Akcora explains, "furthermore, it will hold various advantages such as high mechanical stability since the material is composed of inorganic particles, contain low ionic content in polymer chains and has low water uptake. The nanomaterial design is planned to control ion aggregation and to study confinement of ionic liquids within nanostructured particles to enhance ionic mobility and to unravel ion transport mechanisms."
The NSF grant will not only enable her to pursue this line of research; it will train graduate, undergraduate students and teachers about materials for energy applications as part of a Nanoscience Educator workshop. "The workshop will be organized for high school science teachers in New Jersey/New York area, aiming to help teachers to integrate nanoscale science concepts and applications into their courses," Akcora explains.