From Head to Toe, Stevens Researchers Are Keeping People Moving and Having Fun
Biomedical and mechanical engineers are developing innovative technologies and methodologies to help people find more effective and engaging ways to recover from, and in some cases, prevent issues while walking and conducting other activities of living
Whatever your age, physical condition, or medical situation, at some point, you’re likely to experience a short- or long-term situation that makes it difficult, painful, or even impossible to walk, pull on a T-shirt, hold your smartphone, or otherwise go about your daily routine. Knowing this, maybe you’re interested in being proactive and minimizing your chances of having your movement hindered. Either way, biomedical and mechanical engineers at Stevens Institute of Technology have got your back – and your hands, arms, shoulders, legs, and feet. Their innovative research is uncovering novel approaches to helping people with injuries regain and maintain function – and have fun doing it.
More engaging physical therapy is at hand for those with severe neurotraumas and lost limbs
Having a stroke, suffering a spinal cord injury, or losing a limb can be devastating, and literally adding insult to injury, the resulting lack of independence can drastically hinder both the individual’s physical and emotional rehabilitation and recovery.
Stevens biomedical engineering professor Raviraj “Ravi” Nataraj and his team are working to change that by helping people with these severe neurotraumas and other injuries improve their hand reach and grasp through innovative approaches to physical rehabilitation.
Nataraj’s Movement Control Rehabilitation (MoCoRe) Lab is dedicated to investigating how to optimize this functional rehabilitation through a sense of agency (the feeling of control over actions), sensory-guided training using vision and touch, and computer simulations.
“We develop instrumented wearables and virtual reality environments that improve patient engagement and progress during rehabilitation training,” Nataraj said. “This approach is novel for rehabilitation because it is more user-centered.”
The lab’s first major device platform is an instrumented glove that computes the ideal secure grasp from onboard force and flex sensors, determines when the patient achieves that secure grasp on an object, and provides feedback through sounds, lights, and vibrations on the glove, or through its compatibility with virtual reality (VR) environments.
“With the inclusion of virtual reality, that is synchronized with the real environment,” Nataraj explained, “we hope to provide sensory feedback that further improves agency through the timing and variations of that feedback. These variations may include speed of the virtual avatars, personalized messages or pictures that serve as rewards, or color changes that amplify the person's recognition of secure grasp.”
Studies show the more physical therapy one does, the more benefits one receives, but it’s a rigorous process, and it’s hard to get people to stick with it. “We’re trying to make it more fun in specific, scientifically validated ways that engage users so they can learn to move better and faster,” Nataraj said. “We have preliminary indications this approach can accelerate improved performance of grasp-and-place tasks, even with healthy people, and we expect people with functional impairments will also benefit. In the near future, we will be testing our platforms with people who have spinal cord or traumatic brain injuries.”
The next major platform underway at the MoCoRe lab is a VR+brace system designed to improve the muscle activation control of a virtual prosthetic arm. The system restricts the upper body while providing variations in the visual and vibration-haptic feedback features during training that improves control.
“The goal is to improve training performance and help users generate more distinct muscle activation patterns for better directional command of the virtual device,” Nataraj explained. “We believe this improved ability to gain the strength and dexterity to direct a virtual arm based on muscle control will allow people, once removed from our training system, to better control their own arm or a prosthesis or powered exoskeleton.”
While helping users improve their ability to move their arms and hands is the obvious goal, Nataraj and his team are aiming for a much deeper impact.
“The crux of what we’re trying to do is to help people feel more engaged in the process,” he said. “We’re big on agency – giving people the perception of better control. It’s all about the human element – not just whether they can restore function, but whether they can also feel in control of that function so they can gain greater independence. It could be the key to unlocking more efficient rehabilitation, and opening up a whole new world to making people’s lives better.”
In addition to teaming up with the world-renowned Cleveland Clinic, where Nataraj did his postdoctoral research, the lab has received grants from the New Jersey Health Foundation and Department of Veterans Affairs, with clinical collaborations at the Kessler Foundation and the James J. Peters Bronx VA Medical Center.
Nataraj’s Ph.D. students are also gaining attention for their work: Sean Sanford was awarded the Stevens Excellence Doctoral Fellowship this year for his dissertation project titled "Multi-sensory training to optimize myoelectric control," and Mingxiao Liu recently gave a podium presentation on "An Instrumented Glove to Induce Agency-based Performance of Secure Grasp" at the American Society of Biomechanics.
‘Shocking’ research opens the ‘gait’ to better function for people with Parkinson’s Disease
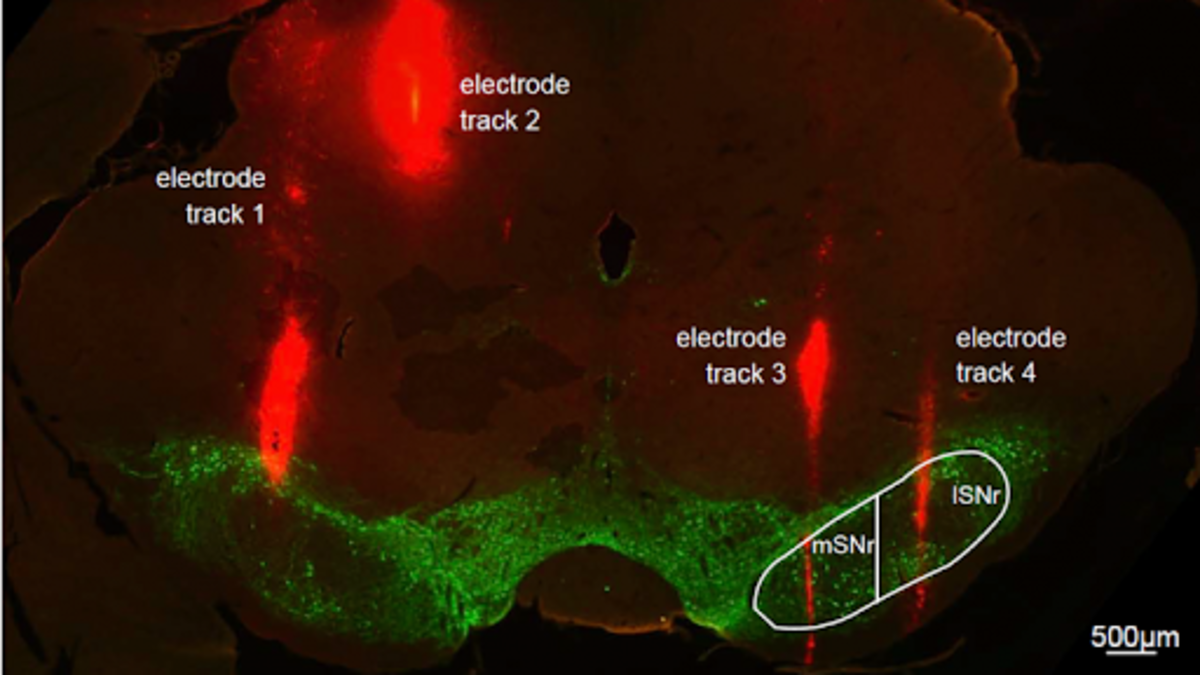
A tiny electrode narrower than a human hair and a part of the rat brain about the size of a grain of rice are showing massive potential to improve the lives of people with Parkinson’s Disease.
Stevens assistant professor of biomedical engineering George McConnell, as well as undergraduate and graduate students at his Laboratory for NeuroInnovation, are working on promising research to help guide clinicians in helping people with Parkinson’s correct associated aberrant brain circuitry to walk and function better. This research has been funded by groups such as the National Institutes of Health and the Branfman Family Foundation.
McConnell and his team implant fine (75-micron-diameter) electrodes deep into targeted areas of Parkinson’s-like brains of rats. They then apply Deep Brain Stimulation (DBS), a U.S. Food and Drug Administration-approved surgery to implant a device that acts like a pacemaker in the brain. The researchers use DBS to deliver tiny electrical impulses, pulsing the brain at various amplitudes, durations, and rates, then studying how the rats’ behavior and neural activity change. The goal is to leverage engineering techniques to quantify patterns of brain activity during DBS therapy to inform the design of DBS 2.0.
“Despite how well DBS works, we do not still fully understand why or how it works,” McConnell explained. “My lab has been investigating DBS at the substantia nigra pars reticulata (SNr), which is associated with locomotion, as a novel target to treat gait dysfunction. It’s close to the subthalamic nucleus (STN), the main DBS target used to treat tremor, so the hope is that in humans, the neurosurgeon may be able to lower the stimulating electrode slightly to treat both tremor and gait dysfunction.”
Remarkably, after use in more than 100,000 patients, just seconds after the stimulator is turned on, DBS has been shown to eliminate tremors, restoring as much as five years in terms of quality of life. The trick, of course, is putting the wire in the right place.
“Hanyan Li, a Stevens Ph.D. student who has since graduated, developed the Stimulus Pulse Aligned Coherence Analysis in Evoked Recordings, or SPACER, technique, which is now patent-pending,” McConnell said. “SPACER allows us to get a sense for where we are in the brain while we lower the electrode, and when we reach the ideal region to affect Parkinson’s Disease symptoms. Instead of just listening passively to the brain, SPACER lets us interrogate the brain by stimulating it at much lower DBS frequencies than usual – less than 1 Hertz, rather than more than 100 Hertz – and analyzing the brain’s response in between pulses. The goal is to create a color map that neurosurgeons can use to reliably place the electrode in the ‘sweet spot’.”
According to the Parkinson’s Disease Foundation, more than 10 million people worldwide live with Parkinson’s Disease. They’re the ones who keep McConnell motivated.
“Gait dysfunction is a particular focus because it’s found in most people with Parkinson’s Disease and worsens as the disease becomes more advanced; it increases the risk of falls; and it’s notoriously difficult to treat with current pharmacological approaches” he said. “Years ago at the Parkinson’s Policy Forum, a woman stopped by my poster presentation and told me she was so glad someone was working on this. She had a friend who also had PD, and who had fallen down a flight of stairs and died just a week before. It really hit home that we need better treatments. When we have a good understanding of the pathophysiology, biomedical engineering has a significant potential to profoundly help people with better, life-changing solutions.”
Wearable technology is a step in the right direction for both patients and therapists
When someone is recovering from a brain injury such as a stroke, the rigorous physical therapy required to overcome the resulting muscle weakness and partial paralysis and regain motor function doesn’t just take a toll on the patient – it’s a physically demanding, labor-intensive process for the physical therapist as well, and it often requires more than one therapist.
Damiano Zanotto, Stevens assistant mechanical engineering professor, and his team are working to make such therapy more efficient and effective for both patients and therapists with an innovative robotic lower-leg exoskeleton that recently was granted a five-year, $597,471 CAREER Award from the National Science Foundation (NSF).
The “Reinforcement-Learning Assist-as-Needed Control for Robot-Assisted Gait Training” project brings adaptive, assist-as-needed controllers to a powered ankle brace.
“The idea is to develop a powered, individualized orthosis that can automatically adapt the level of response to assist in gait rehabilitation,” Zanotto said. “The therapist sets up the device, which performs the repetitive therapy tasks. This frees the clinician to supervise multiple patients, so the same workforce can deliver more intense, frequent, and successful rehab sessions.”
Properly evolving the amount of robotic assistance is key to success.
“To help people walk as they did before the injury, the idea is to find the sweet spot between the correct execution of the walking cycles and sequence of steps, and the level of assistance provided by the robot,” Zanotto explained. “The robot should only provide what’s strictly required for the subject to perform these tasks, so based on that performance, it has to constantly adjust the torques and observe the effects. We want the subject to eventually get rid of the robot and walk normally without the exoskeleton.”
This is just one of the exciting projects Zanotto and his team are working on at his Wearable Robotic Systems Laboratory, which leverages motor learning principles and mechanical and biomechanical models to develop wearable robotic devices and related control strategies to help people with movement disorders.
The researchers also design unobtrusive, custom, wearable sensors and computational models to accurately quantify movements to support more accessible and reliable tools for early diagnosis and objective assessments of medical conditions affecting the motor function.
“Instead of having the patient fill out questionnaires or perform a standardized set of motor tasks in controlled clinical settings, we use unobtrusive, lightweight, and very affordable sensors to accurately capture biomechanical quantities in real-life environments,” Zanotto said. “Our machine learning models can improve the accuracy and reduce the noise of sensors embedded in devices such as our artificial intelligence-powered, smart insole with our patent-pending SportSole technology. Clinicians can reliably and accurately measure how long each stride is, how far the foot clears the ground, the force placed on the ground, and other important data, all from a little electronic pod in the patient’s sneaker, and in the comfort of a patient’s home.”
This fall, clinical researchers at Columbia University, where Zanotto spent time as an associate research scientist, will be testing the insoles with patients with muscular dystrophies in their living environments, continuously, for one week. Zanotto and his collaborators at Columbia have also used the insoles to screen children who have been identified as at high risk for autism spectrum disorders, which can manifest in subtle differences in temporal and spatial gait parameters. Also in the works is a wearable biofeedback system leveraging the insoles technology to help patients with walking impairments get real-time feedback to improve home-based, self-administered gait rehabilitation exercises.
“Working with patients and clinicians is a great experience,” he said, “because it gives us a whole new perspective that integrates and complements our engineering mindset.”
Research is on the move to help rehab and ‘prehab’
For older adults at high risk for falls, safer walking can literally sound great, thanks to the research Antonia Zaferiou, assistant professor in the department of biomedical engineering at Stevens, and her team are conducting to use sonification and sound biofeedback to train people’s movement patterns.
Zaferiou received a five-year NSF CAREER award providing $822,200 in funding for the “Adaptive Sonification to Improve Balance During Everyday Mobility” project, which incorporates machine learning and wearable technology to identify balance and gait deficits in older adults, and then develop and test personalized auditory cues to improve dynamic balance while walking.
“Sonification is a novel, emerging field that uses expert practices from the entertainment field in how to engage people deeply in sounds and music, and folding these methods into rehabilitation and prehabilitation practices,” Zaferiou said. “People are accustomed to moving with sound, and using sound as cues to things such as recognizing people by their unique footsteps, but in other ways, conveying movement mechanics through sound is quite foreign. In our lab, we’re learning to understand how people perceive sound cues, and to gauge their utility in movement training. In this research, there are more unknowns than knowns, which will keep us motivated for years to come.”
Sensors, both wearable and installed in the ground, measure data such as body rotational speeds, muscle activity, and ground reaction forces. Sonified biofeedback allows participants to use their movements to create sound, which in turn informs and helps them fine-tune their movements. In addition to helping people who are already experiencing difficulties balancing during everyday walking, this approach has the potential to help people train to improve their movement and avoid these issues – and related injuries – in the first place.
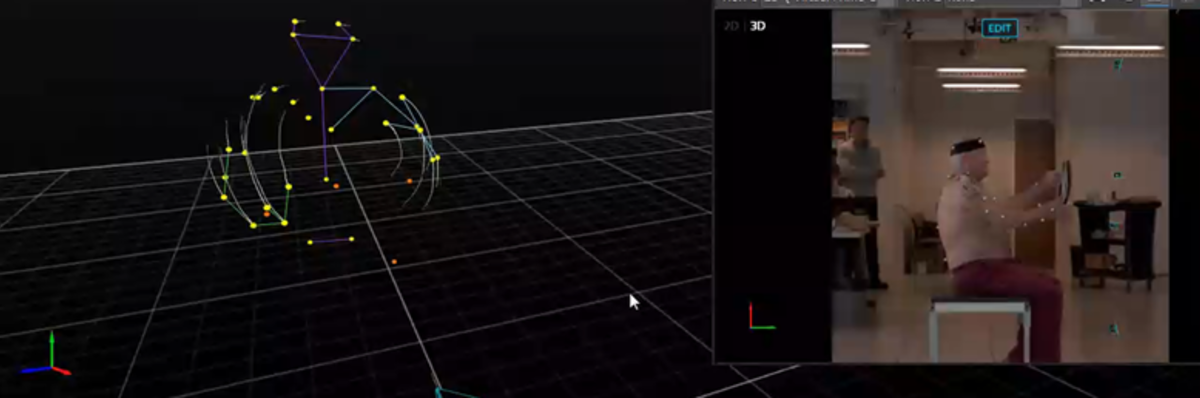
Zaferiou and her team are also putting their shoulders to the wheel to understand how reverse total shoulder arthroplasty affects movement and muscle activation patterns during range of motion movements and activities of daily living. Collaborating with orthopedic surgeons at Rush University Medical Center, where Zaferiou previously taught, and other institutions, they are working to understand how the calibration of tools that measure shoulder movement affects the orthopedic outcome measurements.
“In our study, patients’ muscle and movement patterns are measured before and after the shoulder replacement surgery,” Zaferiou noted. “Sensors placed on the skin help us estimate what’s going on with the bones and joints underneath, but depending on where the sensors are placed and how the movements are measured, results can vary by up to 30 degrees. This makes it difficult to know the details about how the surgery affects movement.”
To measure shoulder movement, Zaferiou uses optical motion capture technology, which leverages infrared cameras that measure the 3D locations of reflective “markers,” or spheres. This technology is also used in creating movies and video games.
“We recently published an article quantifying how much certain outcome measurements are affected by the calibration protocol when using optical motion capture to measure scapula, or shoulder blade, rotations,” she said. “Unknown distances between where we place the reflective marker on the skin and the underlying anatomical bony structure can contribute to measurement errors. That’s why calibration protocols can help. The best practice includes placing a ‘tracking marker cluster’ at the top part of the scapula, which has the least relative motion between the skin and the underlying scapula. We take multiple calibration measurements with the patients’ arms in different locations so we can mathematically relate the cluster to the other scapula landmarks.”
Interestingly, they have found that some measurements are less prone to differences in methodology than others. For example, some measures are within five degrees regardless of calibration, as opposed to up to 20 degrees in variation with other methods.
“This finding gives us a way forward that improves the quality of our shoulder movement measurements,” she explained, “allowing us to better monitor the effects of different orthopedic interventions.” It’s somewhat abstract work with very real benefits.
“Studies show that as many as one in three people over 60 will experience a rotator cuff tear, and if they have symptoms, they can’t brush their teeth, bathe, drive, or reach into a laundry machine without pain,” Zaferiou said. “Last year, I hurt my shoulder, and for three months it was so limiting and so painful – with physical therapy it eventually got better, but it was a wakeup call for how important this work is. In the future, parents and grandparents likely won’t experience such common pain when we better understand the complex movement mechanics of the shoulder, the true benefits of interventions, and the kind of ‘prehabilitation’ that can help us avoid these musculoskeletal issues altogether.”
Stevens performs leading-edge research in diverse and critical disciplines. Bringing together individual experts from across these fields, such as with these projects spanning both the sciences and engineering studies, regularly proves the adage of the whole being greater than the sum of its parts. These powerful collaborations continue to maximize both the intellectual and societal impact of this research.